Forget about building a better mousetrap; make a better battery and you expand the possibilities for renewable energy and cleaner vehicles. Let's see what's happening on the front lines of battery research.
Battery, Heal Thyself
Li-ion is becoming the standard technology for rechargeable batteries, but it's not devoid of shortcomings. These batteries often employ a carbon-based negative electrode. Silicon electrodes would provide a higher energy density (energy per unit of volume), making them more desirable for electric vehicles. The problem is that silicon expands and contracts with recharge cycles, eventually causing the electrode to fall apart, kind of like freezing and thawing of a road surface creates potholes.
Engineers at the University of Illinois are taking a multifaceted approach to this problem. One potential solution is a self-healing electrode that uses a conductive substance embedded into microcapsules. As the electrodes expand, the microcapsules rupture and disperse the crack-filling material.
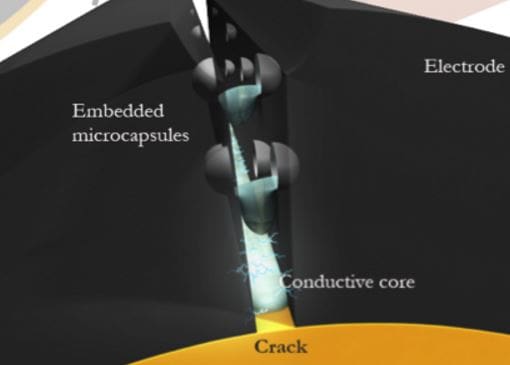
Microcapsules rupture and fill cracks with a conductive material. (Image: University of Illinois)
The same U of I team is working on a self-healing electrode that features dynamic bonding between the silicon nanoparticles and a polymer binder. Early tests have shown that silicon electrodes employing this technology remain stable through several hundred charging cycles.
Dendrite Prevention
One problem that plagues Li-ion batteries is the formation of dendrites – tiny metallic structures that form on one electrode and grow toward the other, causing the battery to eventually short-circuit and possibly catch fire The dendrites easily grow in the liquid electrolyte that's prevalent in Li-ion technology, so researchers developed solid electrolytes, which are stronger. But as any programmer will tell you, when you fix one bug you often create another. As the battery goes through charging/discharging cycles, the electrodes expand and contract, which can damage the solid electrolyte and allow dendrites to form.
Scientists at MIT have examined the cause of dendrite formation and found that previous researchers were focusing on the wrong problem. They determined that it's not the weakness of the electrolyte material that allows dendrites to form, it's the uneven surface.
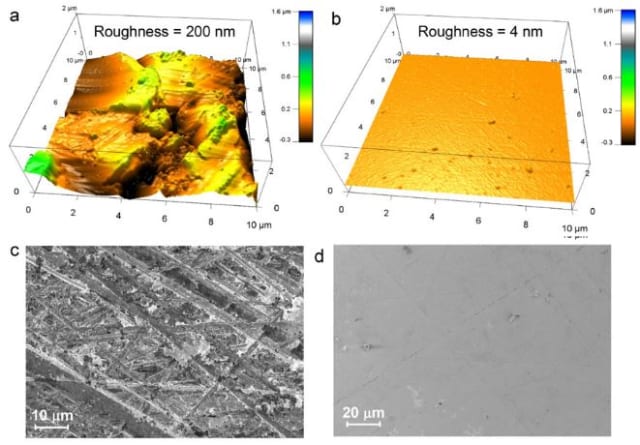
Smooth electrolyte surfaces can prevent dendrite formation. (Image: MIT)
Rough surfaces provide places where dendrites can infiltrate the material, eventually working their way to through to the other side. Engineers have been working on stronger electrolyte materials under the assumption that dendrites will form no matter what, so they need a tougher "wall" to block them. MIT's research shows that with ultra-smooth solid electrolyte surfaces, dendrites can be prevented rather than blocked. Now the question is whether these electrolytes can be manufactured at a reasonable cost. If so, it could open the possibilities for solid-state Li-ion batteries to be used in electric vehicles and renewable energy systems.
It's been more than two centuries since Alessandro Volta invented the "voltaic pile" – the first battery in the modern sense of the word. Since then, chemists, materials scientists, and engineers have tweaked the device's molecules in order to improve performance. Those enhancements will keep the inventor's name on our lips for many years to come, as we see more electric vehicles like the Volt and renewable energy from photovoltaics, both of which store energy in Volta's electrochemical sandwich. Saluti, Alessandro!
article content and image appeared are from Engineering